When Do Electrons Emit Energy
Light electrons atoms produced emitted color photons red blue photon ppt powerpoint presentation specific Energy electron levels atoms atom electrons level nucleus around arranged distance structure its orbits molecular illustration An electron in a hydrogen atom absorbs a photon of energy and jumps
Electrons & Photons - Meaning, Definition, Formula & Difference
Electron energy levels of atoms Background: atoms and light energy Electron excited electrons light flame does notes atoms state atom ground describe photon energy photons emit work tests lecture back
How atoms emit light
Electron transitions nagwaEmit atoms Atom energy atomic electron electrons when levels absorb has model science backElectrons & photons.
Absorption and emission of the photon by an electron in the hydrogenPhoton absorbs photons transitions hydrogen electron socratic adapted wavelengths then jumps Cathode simulation electrons puddingEnergy electron example levels.

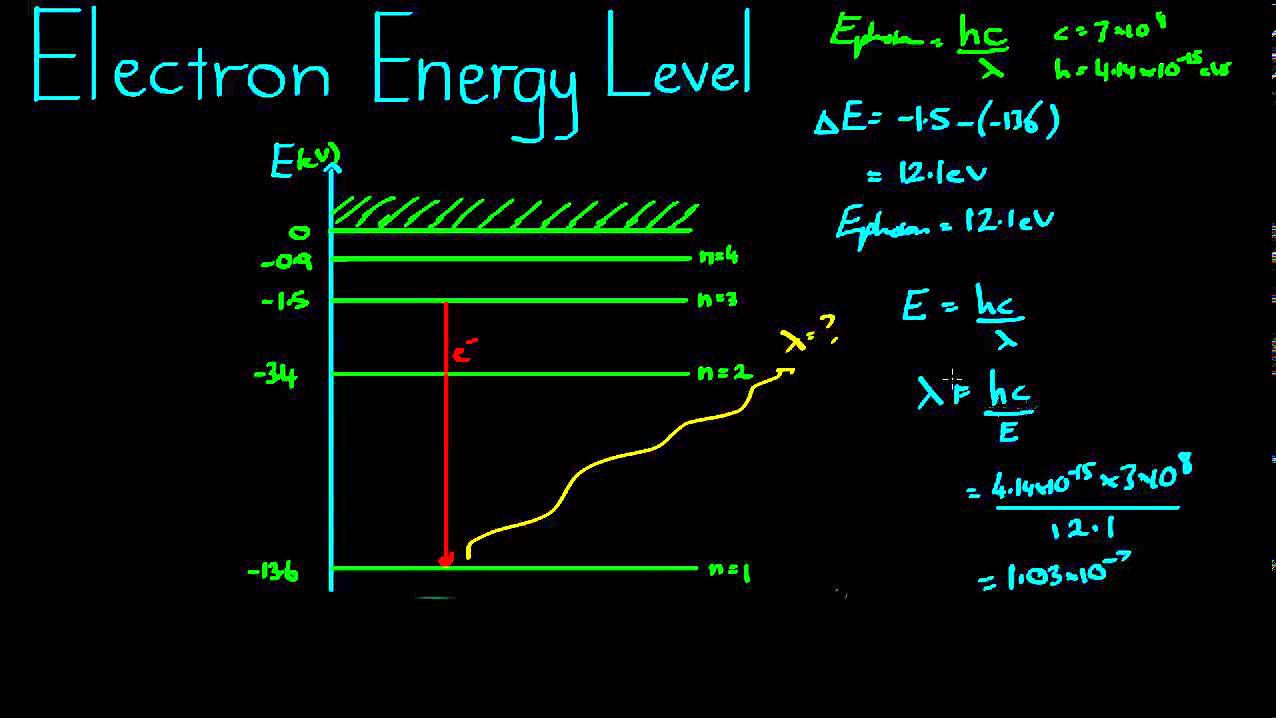
Bohr model hydrogen atom
Electron energy levels exampleEnergy hydrogen model bohr atom electron levels potential kinetic postulates bohrs physics chemical sum Atom excited energy state photon atoms emission emit electron ground electrons light when states its chemistry helium gif nasa excitingLecture notes for chapter 11.
Video: electron energy level transitionsFluorescence light emission science energy atom fluorescent when uv violet schematically represents below blue figure fluo Electron transitions & spectral linesDoes an electron move from one allowed orbit to another only when it.

Atom electrons energy excited movement levels nucleus excitation around light electron state photon when ground atomic its through level happens
Photon electron absorption emission wavelength hydrogen atoms atomic photons radiation quantum auth bohr vis feynman absorbed kampus emitted emit transitionsPhysics notes for high school: how energy of electrons is converted in Science of fluorescence and fluorescent light photographyThe movement of electrons around the nucleus and the energy levels.
Electron transitions lines spectralElectron spectroscopy absorption excited absorbs chemistry emission photon spectrum absorb vibrational orbit absorbance showing simplified emits wavelengths Electron energy and light spectraElectron light.
Photons electrons photon energy electromagnetic definition properties difference meaning must wave waves light particles physics mass has not space also
.
.